In Vivo Replacement
NGS as an ethical alternative to animals in biosafety testing and characterization.


What is in vivo replacement and why is it important?
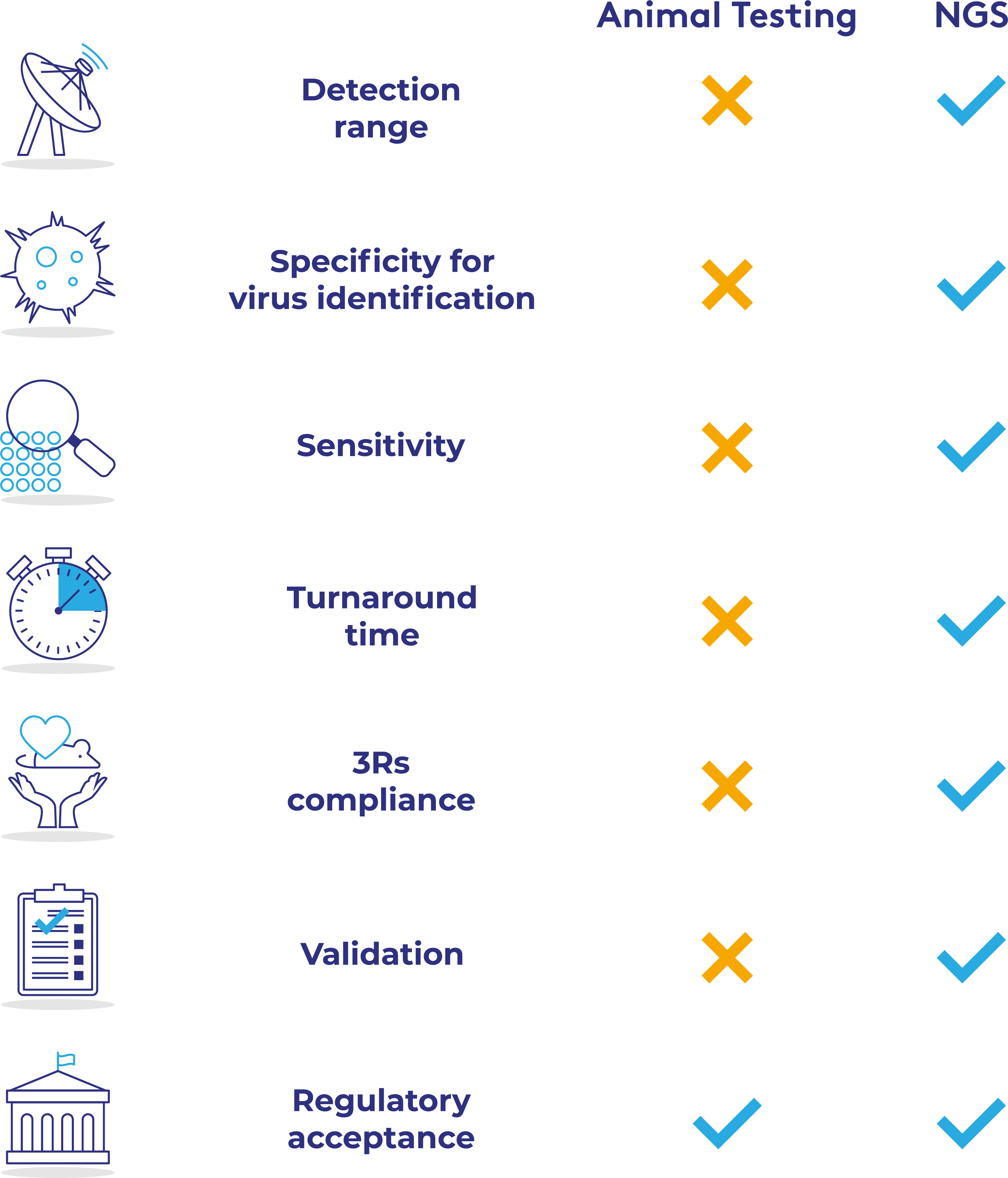
NGS approaches have long been used for the agnostic detection of unknown pathogens both clinically and in commercial biopharmacutical manufacture, especially in contamination investigation. However, the adoption of NGS as a replacement to animal models has been slow due to concerns about regulatory acceptance, as well as the sensitivity of NGS.
In collaboration with Charles River Laboratories, PathoQuest is the first GMP CRO to conclusively show the superiority of iDTECT® NGS assays over conventional in vivo biosafety testing and characterization methods. This, combined with the commitment from regulators to facilitate the removal of animal models from most submissions, means now is the ideal time to begin switching away from in vivo testing.
What we do
- mAbs and recombinant biologics
- Viral vectors
- Cell therapies
- Vaccines
- RNA based therapeutics
- Cultured meat
Benefits of iDTECT® NGS assays
✓ GMP validated – in vivo testing mostly GLP
✓ Fast results – days vs weeks
✓ Much more reliable and robust than animal models
✓ No need to generate neutralizing antibodies
✓ Specific identification of contamination
✓ Meet corporate 3Rs objectives
Our Assays:
- iDTECT® Virome
- iDTECT® Transcriptome
WHY THIS APPROACH
ICH: Draft Guidline for Consultation Q5A (R2) viral safety evaluation of biotechnology products derived from cell lines of human or animal origin
Publication: Historical evaluation of the in vivo adventitious virus test and its potential for replacement with next generation sequencing (NGS)
Publication: Systematic evaluation of in vitro and in vivo adventitious virus assays for the detection of viral contamination of cell banks and biological products
PathoQuest in vivo Brochure
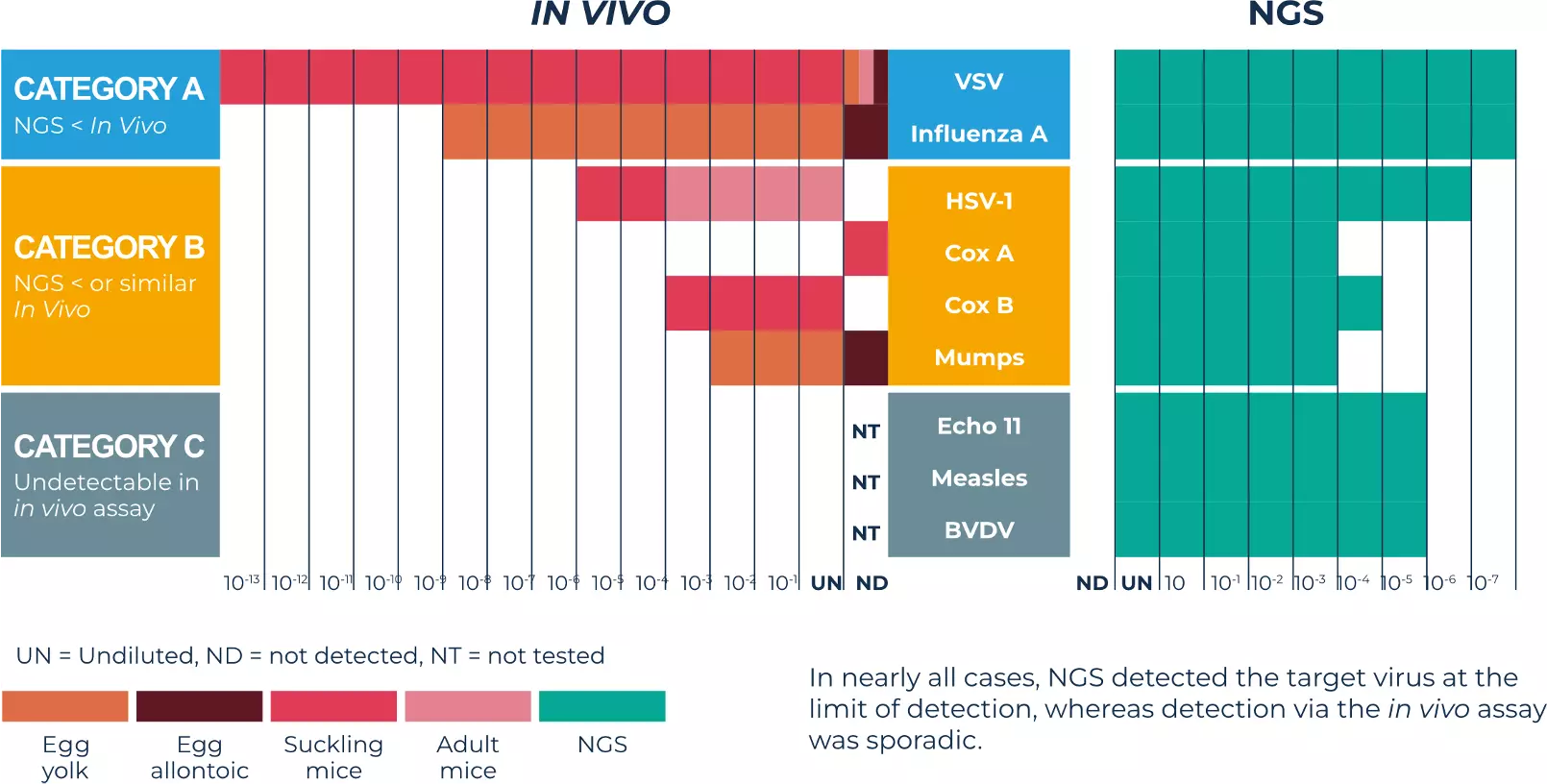
Our comparison data
In vivo assays were performed using suckling mice, adult mice, and embryonated hens’ eggs. For ethical purposes, we chose not to perform all in vivo tests. For each virus category (defined in this publication), only the most sensitive reported ones were used.
Challenges solved
- Difficulty in generating a neutralizing antibody
- Incompatibility of test material with in vivo methods
- Limited sample volume for in vivo characterization
- Meeting corporate objectives to reduce animal use
OTHER SERVICES
Adventitious Virus Testing
Detection of viral contamination within the manufacturing process and beyond.
READ MORE
Integration Site Analysis
Characterisation of genetic modifications for clone selection, genetic stability and lot release
Identity Confirmation
Genetic characterization of viral and plasmid products for release.
READ MORE
Cell Line Characterization
Biosafety screening and stability testing of manufacturing cells.
READ MORE
HLA Genotyping
Characterizing and screening for novel and emerging cell therapies.
READ MORE
Raw Material Testing
Screening of high-risk inputs such as animal products and media.
READ MORE
Contact us
U.S.
466 Devon Park Dr
Wayne, PA 19087
United States
E: contact@pathoquest.com
Sign up for our latest news
France
+33 (0)1 70 82 17 90
Biopark -Bâtiment B,
11, rue Watt
75013 Paris, France
How can PathoQuest help?
U.S.
466 Devon Park Dr
Wayne, PA 19087
United States
France
+33 (0)1 70 82 17 90
Biopark -Bâtiment B,
11, rue Watt
75013 Paris, France
E: contact@pathoquest.com
How can PathoQuest help?
Sign up for our latest news